Germanium Atomic Number
Germanium, categorized as a metalloid in group 14, the Carbon family, has five naturally occurring isotopes. Germanium, abundant in the Earth's crust has been said to improve the immune system of cancer patients. It is also used in transistors, but its most important use is in fiber-optic systems and infrared optics.
- Germanium's existence was predicted before anyone isolated it. This was a triumph for Dmitri Mendeleev in his construction of the periodic table. By 1869, Mendeleev had assembled a crude table of the known elements, arranging them according to their chemical properties and atomic weights. But his table had a number of prominent gaps.
- Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, 'Ge': 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation. The closest noble gas.
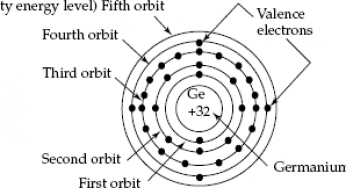
Germanium (atomic symbol: Ge, atomic number: 32) is a Block P, Group 14, Period 4 element with an atomic weight of 72.63. The number of electrons in each of germanium's shells is 2, 8, 18, 4 and its electron configuration is Ar 3d 10 4s 2 4p 2. The germanium atom has a radius of 122.5 pm and a Van der Waals radius of 211 pm.
Germanium Number Of Protons
Introduction
The metalloid was one of the elements predicted by Mendeleev in 1871 (ekasilicon) to fill out his periodic table and was discovered in 1886 by Winkler. In a mine near Freiberg, Saxony, a new mineral was found in 1885. First the mineral was called argyrodite but later when Clemens Winkler examined this mineral he discovered that it was similar to antimony. At first he wanted to name it neptunium, but because this name was already taken he named it germanium in honor of his fatherland Germany.
The position of where germanium should be placed on the periodic table was under discussion during the time due to its similarities in arsenic and antimony. Due to Mendeleev's prediction of ekasilicon, germanium's place on the periodic table was confirmed because of the similar properties predicted and similar properties deduced from examining the mineral.
Germanium Atomic Structure
Characteristics
Like silicon, germanium is used in the manufacture of semi-conductor devices. Unlike silicon, it is rather rare (only about 1 part in 10 million parts in the earth's crust). The physical and chemical properties of germanium closely parallel those of silicon. Germanium (Ge), has an atomic number of 32. It is grayish-white, lustrous, hard and has similar chemical properties to tin and silicon. Germanium is brittle and silvery-white under standard conditions. Germanium under this condition is known as α-germanium, which has a diamond cubic crystal structure. When germanium is above 120 kilobars, germanium has a different allotrope known as β-germanium. Germanium is one of the few substances like water that expands when it solidifies.
| Atomic Number: | 32 |
| Atomic Symbol: | Ge |
| Atomic Weight: | 72.59 |
| Atomic Radius: | 122.5 pm |
| Series: | Metalloid |
| Group: | 14 |
| Density: | 5.353 g/cm3 |
| Specific Heat: | 0.32J/gK |
| Electronic Configuration: | [Ar] 4s23d104p2 |
| Melting Point: | 938.25 C |
| Boiling Point: | 2833 C |
| Common Oxidation States: | 4,2 |
Germanium, a semiconductor, is the first metallic metal to become a superconductor in the presence of strong electromagnetic field.

Isotopes
Atomic Number Chart
The five naturally occurring isotopes of Germanium have the atomic masses of 70, 72, 73, 74, and 76. Germanium 76 is slightly radioactive and is the least common. Germanium 74 is the most common isotope having the greatest natural abundance of the five. Under the condition of being bombarded with alpha particles, Germanium 72 generates stable Se 77.
Chemical Properities
At temperature 250 °C, germanium slowly oxidizes to (GeO_2). Germanium dissolves slowly in concentrated sulfuric acid, and is insoluble in diluted acids and alkalis. It will react violently with molten alkalis to produce [GeO3]2-. The common oxidation state that Germanium occurs in is +4 and +2. Under rare conditions, Germanium also occurs in oxidation states of +3, +1, and -4.
There are two forms of oxides of germanium, germanium dioxide ((GeO_2)) and germanium monoxide ((GeO)). By roasting germanium sulfide, germanium dioxide can be obtained. As for germanium monoxide, it can be obtained by the high temperature reaction of germanium dioxide and germanium metal. Germanium dioxide has the unusual property of refractive index for light but transparency to infrared light.
Applications and Uses
Like silicon, germanium is used in the manufacture of semi-conductor devices. Unlike silicon, it is rather rare (only about 1 part in 10 million parts in the earth's crust). Although it forms a compound, germanium dioxide, just like silicon, it is generally extracted from the by-products of zinc refining.
Germanium is used mainly for fiber-optic systems and infrared optics. It is also used for polymerization catalysts, electronics, and as phosphors, metallurgy, and chemotherapy. Because germanium dioxide have a high index of refraction and low optical dispersion it is useful for wide-angle camera lenses. As stated before, it is an important semi-conductor so it is used in transistors.
